Welcome to the Längst Group
We are part of the Institute for Biochemistry, Genetics and Microbiology and member of the Regensburg Center for Biochemistry (RCB) at the University of Regensburg.
The Längst Group investigates the mechanisms and dynamics of packaging the eukaryotic and viral genetic material into chromatin and the latter's function in disease processes.
This research includes human chromatin, the genetic packaging of Adenovirus and Coronavirus, and chromatin structure dynamics in the Malaria-causing parasite Plasmodium falciparum.
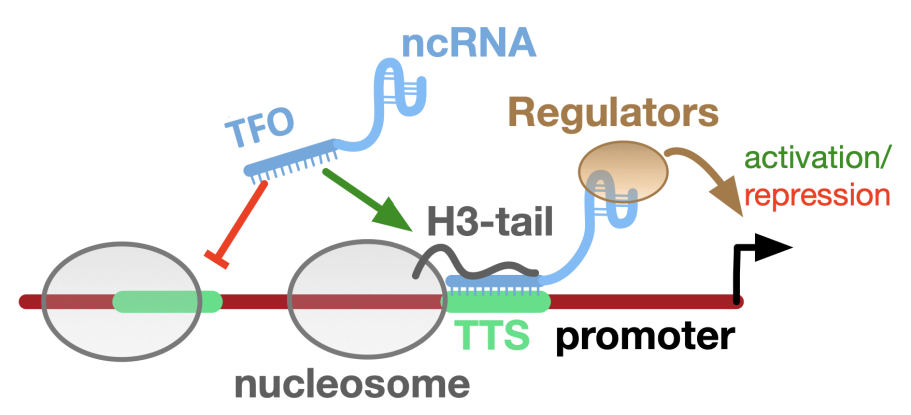
While our fundamental research focuses on chromatin remodelling complexes and non-coding RNAs in nuclear architecture, nucleosome positioning and gene regulation, our applied research relates to inhibiting the dynamics and packaging of the genetic material to identify new drug targets and block cancer, pathogen and viral growth.
Our work on TV
NANO Episode - 24th April 2025
Our Malaria interview starts after 11min.
Shorter film in the ARD Mediathek.
Our work in the press
Detailed interview in Tagesschau.de
Watzlowik et al., Nature (2025) link to research article
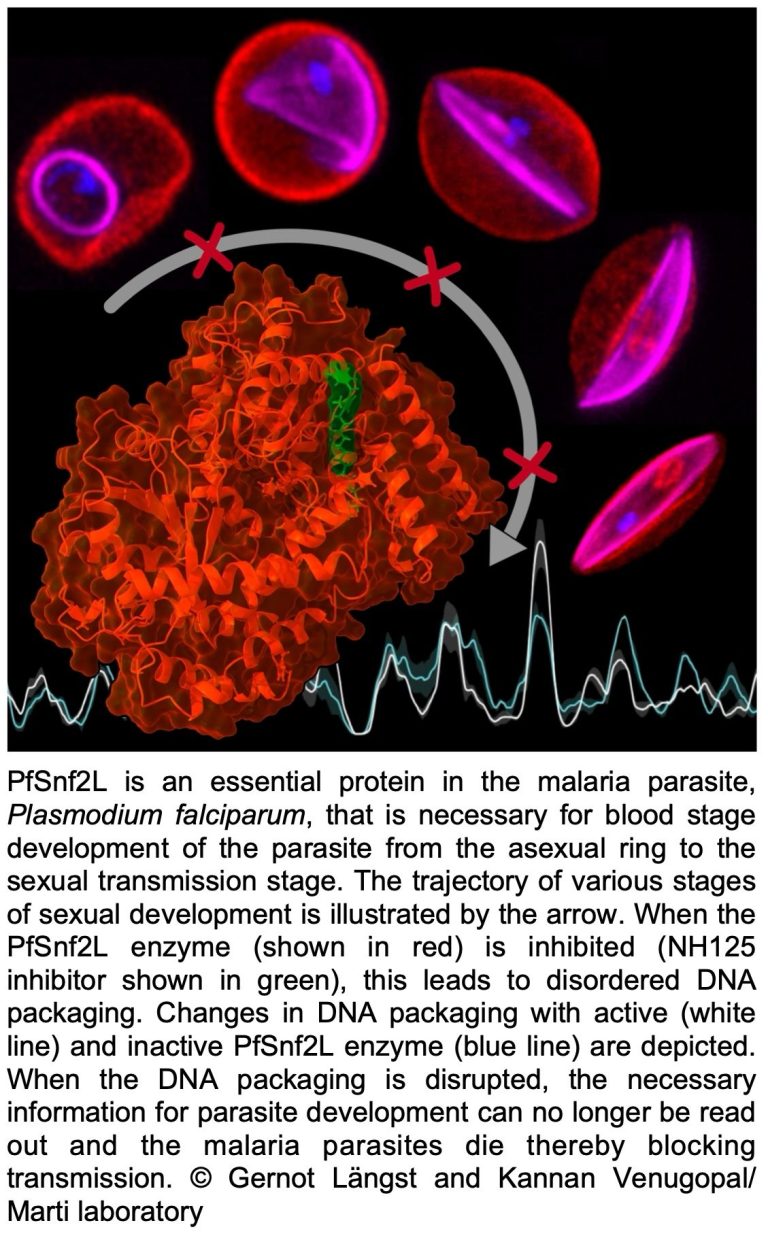
Plasmodium blood stage development requires the chromatin remodeller Snf2L
Malaria is caused by parasites of the genus Plasmodium, which is transmitted to humans through the bite of infected mosquitoes. Plasmodium falciparum, the deadliest of the malaria species, has a highly complex life cycle controlled by precise gene regulation. Understanding these regulatory processes is crucial to specifically combat the pathogen at different stages of development.
Our new study reveals how regulatory proteins modulate genome-wide epigenetic mechanisms to control the precise timing of gene expression in the parasite. We show that the chromatin remodeler PfSnf2L is an essential regulator of “just-in-time” regulation of stage specifically expressed genes. The unique sequence and functional properties of PfSnf2L led to the identification of a highly specific inhibitor that only kills Plasmodium falciparum. A multidisciplinary approach, involving an innovative and unique screening platform for PfSnf2L inhibitors, genetic manipulation of malaria parasites and cutting-edge “OMICs” was used to uncover the role of PfSnf2L. This inhibitor represents a new class of antimalarials, potentially targeting all life cycle stages.
The new findings not only advance basic research but could also offer practical applications. Malaria remains one of the greatest global health threats. In 2022, there were an estimated 247 million infections and over 600,000 deaths, mostly in sub-Saharan Africa. Innovative solutions such as targeting developmental regulation are therefore urgently needed to offer new treatment options.
The study was carried out by an interdisciplinary, multinational team led by Prof. Gernot Längst (University of Regensburg, Germany) and Prof. Markus Meissner (Ludwig-Maximilians University, Munich, Germany) involving researchers from The University of Zürich (Switzerland), The Pennsylvania State University (USA) and the University of Glasgow (UK).
Biophysics Facility News
News
Teaching
We are organising basic and advanced teaching activities. Lectures, seminars, and practical courses are offered for medical students, BSc and MSc students in biology, biochemistry, and molecular medicine.
Graduate School
Gernot Längst is the managing director of the International Graduate School Regensburg (RIGeL). All PhD students of the Faculty NWFIII are members of RIGeL.
Biophysics
Our lab hosts a Biophysics Facility to characterize and quantify all kinds of molecular interactions. The facility is accessible to all UR groups.
Bioinformatics
The Bioinformatic Core Service (GACT) is associated with our group. It is supporting our faculty with advanced bioinformatics regarding high-throughput sequencing analysis.
We are developing Software tools to analyze chromatin structure and to predict sequence specific formation of RNA-DNA triple helices.